The Challenges of Incubating Plates in Environmental Monitoring
Written By: Steven Brimble |
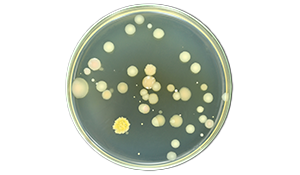
Correct plate incubation is essential for reliable microbial monitoring. Several factors influence incubation success, such as, equipment validation and selecting the appropriate incubation regime. This guide explores the following key factors:
- Why incubation matters: Ensuring accurate microbial monitoring and data integrity
- Equipment validation essentials: Prevent temperature inconsistencies that can compromise results
- Choosing the right incubation regime: Optimise temperature sequencing for reliable microbial recovery
- How incubation time affects results: Prevent overgrowth and maintain accurate colony counts
- How to mitigate risks: Reduce errors through proper maintenance, validation, and operator training
The Importance of Plate Incubation in Microbial Monitoring
Incubation plays a critical role in environmental monitoring, product testing, batch control, and media or disinfection qualification. Any failure of incubation can compromise result validity, leading to costly and time-consuming investigations. Ensuring proper incubation conditions helps maintain data integrity and reliable microbial growth assessment.
Microbial growth and colony morphology are directly influenced by incubation time and temperature, both of which depend on the media used and the chosen incubation regime. Selecting an appropriate incubation approach is crucial for achieving accurate and consistent results.
Ensuring Effective Equipment Validation
Incubators must maintain stable temperatures within the unit. A thorough validation process should include temperature mapping across all shelves and corners in order to identify any out-of-tolerance areas, ensuring only validated zones are used for incubation.
Fan-assisted incubators generally provide uniform heat distribution, but factors such as product load and fan location can impact performance. Poorly positioned fans or excessive product placement can lead to agar desiccation, negatively affecting microbial growth.
Routine maintenance and requalification schedules should be established to ensure incubators are operating as expected. Changes in incubator use or lapses in maintenance can impact microbial viability, leading to inaccurate data. Best practice involves dedicating separate incubators to different functions, ensuring that environmental monitoring plates are housed separately from growth promotion plates to prevent cross-contamination.
Selecting the Appropriate Incubation Regime
Whether selecting a single plate exposed to a dual incubation regime, or using independent plates incubated at different temperatures, needs consideration. Dual incubation generally involves an initial phase at 30-35°C, followed by a second phase at 20-25°C, (or vice versa) with the total incubation period spanning several days. Dual incubation regimes require careful validation as the sequence of temperature exposure must consider the typical microbial flora of the environment being assessed. The movement of plates from one incubator to another should also be a consideration, ensuing no plate integrity issues are introduced as part of this process.
The risk of over-colonisation must also be considered as spreading or fast-growing bacteria and fungi can outcompete or suppress other microorganisms, thereby compromising results. Choosing the appropriate temperature sequence reduces this risk, ensuring representative microbial recovery.
The Impact of Incubation Time on Results
The duration of incubation and the timing of plate inspection significantly influence data accuracy. Plates incubated for five days may require daily monitoring to capture events such as overgrowth by dominant organisms, which can impact on the ability to record accurate counts, as well the ability to correctly identify bacteria seen on the plate.
Incubation time also affects colony morphology. For example, Staphylococcus aureus and Staphylococcus epidermidis look different at 24 hours compared to five days. Additionally, variations in media formulations, such as TSA versus TSA with lecithin and tween, can alter microbial morphology.



Incubation protocols should also account for weekends and public holidays, when routine inspections may not be possible, ensuring incubation periods do not extend beyond validated limits, maintaining data integrity.
Mitigating Incubation Risks
While incubation may seem like a routine step, its significance in microbial monitoring should not be underestimated. Proper maintenance, validation, and adherence to best practices reduce the risk of incubation failures and data inaccuracies.
Operator training on colony morphology changes due to incubation conditions also supports effective root cause analysis and corrective actions in response to out-of-trend data. Implementing structured incubation protocols and rigorous equipment validation enhances data reliability and ensures high-quality microbiological monitoring standards.
For more information on AnalytiChem's range of Redipor prepared media products, both off-the-shelf and custom formulations, contact our team.



